Exploring the Potential of Mesenchymal Stem Cells in
Long COVID and COVID Vaccine Injury
There’s no arguing that the COVID-19 pandemic dealt a tsunami-like blow to all of humanity. Death, disability, lockdown, job loss, economic collapse – and that was just the first two years. Following was a rise in depression, drug overdoses, alcoholism, and domestic abuse. Suicide rates among our young people were at an all-time high, children were left in devastating educational lapse, their families in poverty, homelessness… the destruction touched everyone. For a time, cases of mild infection and the promise of life-saving vaccination provided hope that the whole nightmare might soon come to an end. Sadly, even those often came with a heavy price. As we began to dig out from beneath the rubble, two new seemingly insurmountable catastrophes emerged:
Vaccine Injuries & Long COVID.
“Long COVID, the condition where symptoms that surface after recovering from COVID-19linger for weeks, months, or even years, is still a mystery to doctors and researchers.”-Yale Medical
Fatigue, shortness of breath, heart palpitations, cognitive issues and joint pain are common manifestations, but more than 200 symptoms have been identified affecting multiple organ systems. It is estimated that at least 10% of severe COVID infections result in persistent debilitation. Further, research suggests similarities with other viral-onset illnesses including ME/CFS (myalgic encephalomylelitis/chronic fatigue syndrome) and POTS/dysautonomia (postural orthstatic tachycardia syndrome). Current treatments often provide limited if any relief. Patients with severe initial infection seem to be at the highest risk for developing long COVID, but interestingly, even those who reported mild initial infection went on to develop severe long COVID symptoms. “Many people have reported [COVID vaccine] side effects—such as headache, fatigue, and soreness at the injection site—that are generally mild to moderate and go away within a few days.” –Centers for Disease Control.
The healthcare community now faces the challenging task of managing these novel disease states – ones that are somehow caused by or seemingly associated with exposure to the novel spike protein. I believe that to effectively address long COVID symptoms and mitigate vaccine injuries, a paradigm shift towards therapies that harness the body’s own self-repair mechanisms is essential. One such process that holds promise is “autophagy,” which is the body’s innate repair mechanism. Fasting, intermittent fasting, exposure to temperature extremes (ice bath, infrared sauna), resveratrol, metformin, and rapalogs, are all therapies that can promote the body’s self-healing process. By doing so, these treatments clear out damaged and senescent cells, repair intracellular machinery, and restore normal physiological functioning. Another crucial aspect of damage control is immune system modulation. Immunomodulatory therapies such as vitamin D, low dose naltrexone (LDN), and thymic peptides, have shown success in restoring proper immune system function. Recently, a novel intervention has emerged that has demonstrated the potential to do both upregulate autophagy and optimize immune system function: Mesenchymal stem cell therapy (MSCs).
Mesenchymal Stem Cell Therapy
What Are MSCs & How Do They Work MSCs were first discovered and characterized by Dr. Arnold Caplan, a Professor of Biology and the Director of the Skeletal Research Center at Case Western University in Cleveland, Ohio. Mesenchymal stem cells are multi-potent stem cells capable of differentiating into various cell types, including bone, cartilage, and fat cells. Since his original publications, Dr. Caplan stated he wished he would have named these cells ‘medicinal signaling cells’ instead of ‘mesechymal stem cells’, because it was that it was their cell to cell signaling – and not their differentiation capabilities – that drove their regenerative properties. In the lab, MSCs can be manipulated into specific cell lines, but when introduced to a broken human body, they seek out any damaged tissues and get to work on directing repair pathways. Furthermore, MSCs exhibit potent immunomodulatory properties, which makes them a promising candidate for treating inflammatory diseases such as long COVID and vaccine injury. In essence, MSCs teach the body how to fix itself.
Stem Cells 101 Generally speaking, there are two different types of stem cells: embryonic stem cells and adult stem cells. Let’s just get this issue out of the way first – embryonic stem cells are 100% unethical to use for regenerative purposes, 100% too undifferentiated to work for this indication, and 100% ineffective for regenerative medicine purposes. I have never even seen an embryonic stem cell. Let’s move on.
HSCs: ‘hematopoietic stem cells’ (ie those cells used for cord blood banking and bone marrow transplantation)
MSCs: ‘mesenchymal stem cells’ (ie those that repair skeletal tissues, cartilage, bone and bone marrow fat)
HSCs are not used for regenerative purposes – it is the MSCs that do the heavy lifting, so from here on out, we’ll just be talking about these.
Where Do MSCs Come From? MSCs are kind of like your in-laws (not you, Ellen.). You can run into them just about anywhere – but only in small numbers. From a practical standpoint, high concentration MSCs are only found in in a few places: bone marrow, adipose or fat tissue, and postnatal or placental tissue. Placental tissue is just what you think it is – the tissue that is normally discarded following a live birth. When used for regenerative purposes, this tissue has to pass a rigorous screening process. Donors are all live, cesarian section births from non-vaccinated mothers.
For regenerative purposes, you can use your own stem cells (autologous) or someone else’s (allogeneic).
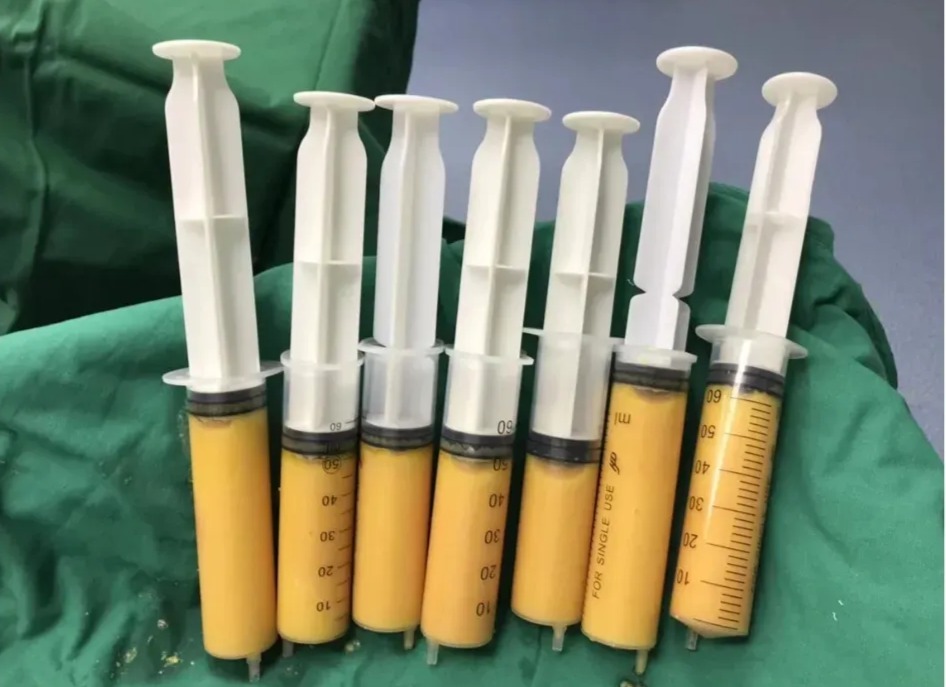
Autologous Stem Cells
You can certainly use your own stem cells, and many providers do. First, they need to be harvested using liposuction or a bone marrow biopsy – and they come out looking pretty much like what’s in those syringes. (Gross – I’m not a fan).
How to MSCs work? Immune System Regulation: MSCs have the ability to modulate the immune system, reducing the exaggerated inflammatory response observed in long Covid and Covid vaccine injury cases. By releasing anti-inflammatory molecules, they help in dampening cytokine storms and tissue damage.
Tissue Repair and Regeneration: MSCs play a vital role in tissue repair and regeneration. Administering MSCs in long COVID patients may aid in healing damaged tissues, potentially leading to improved lung function and overall recovery.
Potential efficacy in Vaccine Injury: Some individuals may experience adverse reactions to the novel Covid vaccines. MSCs offer potential benefits in managing vaccine-induced inflammation and promoting tissue repair, potentially minimizing the impact of such injuries.
Clinical Trials and Research: Numerous clinical trials are underway to explore the safety and efficacy of MSCs in treating long COVID and COVID vaccine injury. Preliminary results have been promising, but more extensive studies are needed to establish their widespread use.
Ethical Considerations: As with any medical intervention, ethical considerations must be addressed regarding MSC therapy and informed consent obtained by all parties involved. Safety, donor selection, and informed consent are critical factors in ensuring the responsible use of this and all cutting-edge therapies. Lack of regulatory oversight and unscrupulous providers make it crucial that patients looking into this treatment do their homework. Ethical biologics companies should be registered by the FDA under an IND or IRB. The FDA currently states that this tissue can be no more than ‘minimally manipulated’ and must be administered for ‘homologous use’. The FDA does not allow stem cell therapy to be marketed for use as a treatment or cure for long COVID or vaccine injury.
The potential of MSC therapy in addressing the lingering effects of long COVID and mitigating COVID vaccine injuries is a hopeful prospect. Although not yet recognized as an approved therapy for these conditions (none currently are), continued research and clinical trials will pave the way for a deeper understanding of MSC therapy, potentially offering relief and improved quality of life for the millions of affected individuals who are still suffering from these catastrophic injuries.
References
What Happens When You Still Have Long COVID Symptoms?
https://www.yalemedicine.org/news/long-covid-symptoms
Yale Medical
www.yalemedicine.org
Selected Adverse Events Reported after COVID-19 Vaccination https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9538893/
Long COVID: major findings, mechanisms and recommendations
https://www.nature.com/articles/s41579-022-00846-2
Introduction to stem cells and regenerative medicine
https://pubmed.ncbi.nlm.nih.gov/23257690/
Human mesenchymal stem cell therapy in severe COVID-19 patients: 2-year follow-up results of a randomized, double-blind, placebo-controlled trial
https://www.thelancet.com
Stem cells: past, present, and future
https://pubmed.ncbi.nlm.nih.gov/34963099/
Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial
https://pubmed.ncbi.nlm.nih.gov/34963099/
Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial
https://pubmed.ncbi.nlm.nih.gov/33568628/
A prospective study on myocardial injury after BNT162b2 mRNA COVID-19 fourth dose vaccination in healthy persons
https://pubmed.ncbi.nlm.nih.gov/36097844/
Nearly 100,000 more deaths involving heart conditions and stroke than usual since pandemic began. https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2023/june/100000-excess-deaths-cardiovascular-disease